

(Like iodine, it would be expected to accumulate in the thyroid gland.) Yet astatine is thought to be more metallic than iodine and is classified as a metalloid.
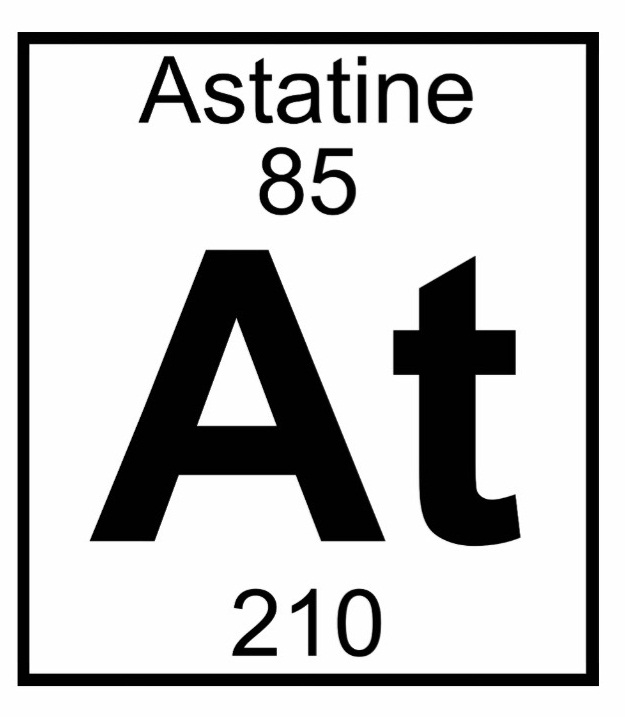
In addition, it lies in period six, between polonium and radon.Īccording to experiments done with a mass spectrometer, the chemical properties of this highly radioactive element probably resemble those of the other halogens, especially iodine. In the periodic table, astatine is located in group 17 (former group 7A), the halogen family, below iodine. An earlier name for the element was alabamine (Ab). They found it during experiments in which they subjected bismuth to a barrage of alpha particles. MacKenzie, and Emilio Segrè at the University of California, Berkeley. This element was discovered in 1940 by Dale R. The name astatine was derived from the Greek word αστατος (astatos), meaning "unsteady." Long before it was discovered, Dmitri Mendeleev had predicted its existence based on his analysis of the periodic table. These isotopes can then be separated from the bismuth by a process of distillation, which involves heating the mixture in the presence of air, and condensing the vapors in a separate container. This method generates the relatively long-lived isotopes 209At, 210At, and 211At. It is produced in nature by the radioactive decay of uranium and thorium, and it is therefore present in trace amounts in minerals of these elements.Īstatine can be artificially produced by bombarding bismuth with energetic alpha particles. The total amount of astatine in the Earth's crust has been estimated to be less than one ounce (28 grams) at any given time-corresponding to no more than one teaspoonful in volume.

Various compounds of astatine have been prepared in minute amounts, and the possibility of their use for nuclear medicine is being studied. All its isotopes are radioactive, and a few of them are produced by the natural radioactive decay of uranium-235 and uranium-238.

Its chemical properties appear to resemble those of iodine. It is a member of the halogen family of elements and is the heaviest halogen. There, the radiation it gives off would kill cancer cells in the gland.Astatine (chemical symbol At, atomic number 85) is the rarest naturally occurring chemical element. When swallowed, the astatine would go to the thyroid. If so, it could be used to treat certain diseases of the thyroid, such as thyroid cancer. One may speculate that it would react similarly to iodine, and following the periodic trend, astatine would presumably sublimate into a dark purple or blue gas, and have a dark metallic luster when solid.Īstatine is far too rare to have any uses, but some researchers think that astatine would behave like iodine. Not much is known about the physical properties of astatine because of its rarity and short half-life. It may be prepared in the lab, but still only in very small quantities. Astatine is extremely rare in nature, and in fact, there is only about an ounce (about a teaspoonful) in the earth's crust at any one moment.


 0 kommentar(er)
0 kommentar(er)
